Are plasmid DNA issues impacting your AAV program delivery?
The manufacture of plasmid DNA for AAV production can create significant challenges, including scalability, fidelity, mis-incorporation of bacterial sequences such as antibiotic resistance genes, high costs and long lead times for GMP production.
Replacing plasmid DNA with enzymatically produced DNA in your AAV production eliminates many issues around complex or unstable sequences, alongside improved speed to manufacture, safety and scalability.
Many biotech and pharma companies focused on advanced therapies are taking advantage of enzymatic DNA such as Touchlight’s doggybone DNA (dbDNA™) to deliver rapid AAV production using less DNA, with no impurities.
What is dbDNA?
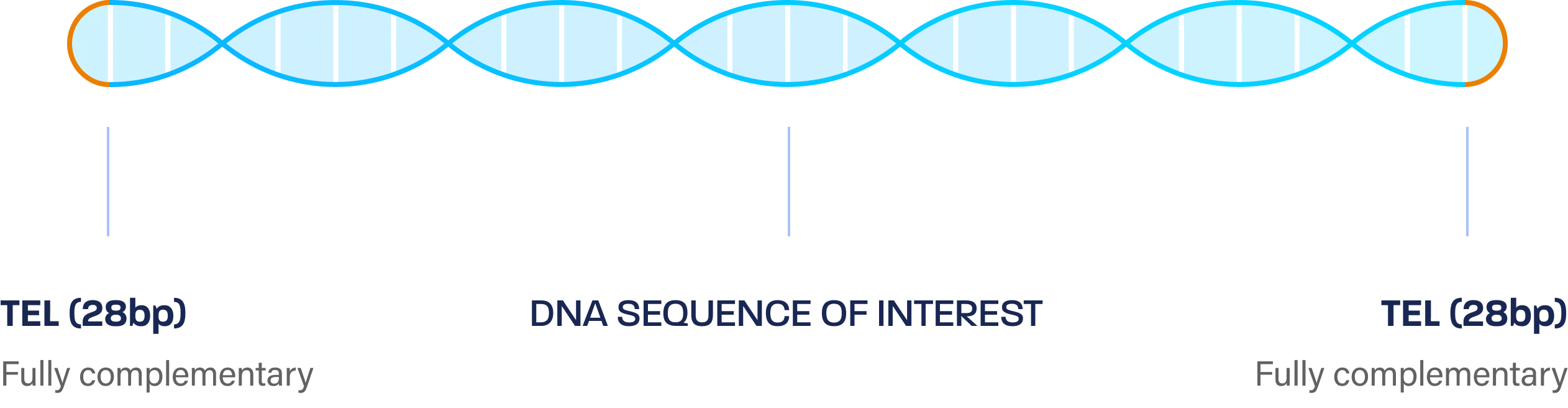
- dbDNA is a linear, double stranded, covalently closed DNA vector which is produced through an enzymatic manufacturing process.
- It can incorporate genes of interest up to 20kb making it very flexible to support a range of genetic medicines.
- The cell-free process avoids selective pressure often associated with plasmid instability, while also delivering rapid and reliable GMP DNA.
How is dbDNA made?
With our proprietary enzymatic DNA production process, we can rapidly manufacture multiple grams of DNA using benchtop, disposable equipment.
How dbDNA can help you.
- Manufactured faster than plasmid - dbDNA limits generational instability by utilising a cell-free enzymatic production process. Enzymatic manufacturing enables scalable GMP DNA production in weeks rather than the months taken to source, make and release plasmid DNA.
- Amplifies unstable sequences (e.g., ITRs) with high fidelity.
- Eliminates packaging of bacterial sequences - Excludes bacterial sequences, including antibiotic resistance genes, avoiding regulatory challenges.
- Reduces E. coli-related impurities - Endotoxin, host cell protein and nucleic acid.
- Scalable - Manufactured using benchtop equipment with a significantly smaller footprint than-fermentation based production.
- Reduced costs at scale - The cost of dbDNA production is predominantly driven by materials, and so the cost of DNA is significantly reduced at scale, while a higher copy number per gram than plasmid means less DNA is needed.

Access dbDNA in different ways.
- Off-the-shelf catalogue products for initial evaluation.
- AAV Constructs
- CMV-Lux-2A-eGFP Transgene
- Rep2Cap2
- AdHelper
- AAV Constructs
- Conduct a feasibility assessment with the supply of mg of dbDNA of your custom sequence of interest. This can be a quick and easy way to assess your product in the dbDNA platform.
- Toxicology and cGMP material supply. Flexible supply and development to meet your scale and timeline.

A perfect partnership to deliver success.
At Touchlight, customer centricity is central to how we operate and communicate. The highest quality of scientific support and project management is at the core of every development program.
- Collaborative culture – we see our clients as partners, always treating them with the upmost respect and courtesy.
- Agile and adaptive – we ensure your project needs are met, on-time and on-budget, even in periods of change.
- Committed to supporting your goals – we are focused on delivering your project as if it were our own.
Learn more
Find out more about our work and research into AAV in our knowledge centre.
Get in touch
If you want to learn more, or simply discuss your AAV or advanced therapy program then please get in touch.



